- 1 HA-BE
- 2 ADMIXTURES
> PRODUCT PORTFOLIO
> Accelerators
> Additives for Concrete
> Admixtures for Mortar & Screed
> Air Entraining Agents
> Curing Systems
> Foam Forming Admixtures
> Hydrophobic & Water Resisting Admixtures
> Plasticizers for MCP
> Plasticizers for Precast & Ready-Mix
> Release Agents
> Retarders
> Special Technologies & Solutions
> Stabilisers
> Superplasticizers
> Swelling Concrete Admixtures> SECTOR
> Manufactured Concrete Products
> Precast Concrete
> Ready-Mix Concrete
> Building Constructors
> Concrete Traffic Areas
> Tunnel & Underground Constructions> OUR BRANDS
> ANTIPOR
> CELVOLIT
> CURING
> DURAHIT
> EMSAC
> PANTAPOR
> PANTARHIT
> PANTARHOL
> PROTECT
> STABILISIERER
> VORMIOL - 3 SURFACE PROTECTION
> PRODUCT PORTFOLIO
> Concrete Optimisation & Plasticizers
> Concrete Surface Deactivator
> Hydrophobic & Water Resisting Admixtures
> Impregnations (colour neutral)
> Impregnations (colour deepening)
> UV- Curing Coating Systems
> Care & Cleaning Products> SECTOR
> Manufactured Concrete Products
> Precast Concrete
> Ready-Mix Concrete
> Architectural Concrete - 4 CONCRETE COLOURS
- 5 FIBRES
> PRODUCT PORTFOLIO
> Polypropylene Fibres | Micro Fibres
> Polypropylene Fibres | Macro Fibres
> Steel Fibres> SECTOR
> Concrete Traffic Areas
> Manufactured Concrete Products
> Precast Concrete
> Ready-Mix Concrete
> Shotcrete | Sprayed Concrete
> Tunnel Segments> REQUIREMENTS & SPECIFICATIONS
> Authority approved Fibres
> Fire Resistance
> Improvement of Impact Resistance
> Optimisation of Green Strength
> Reduction of Shrinkage Cracks - 6 NEWS
- 7 REFERENCES
- 8 CONTACT


PROTECT AGAINST EFFLORESCENCE
PROTECT reduces concretes ability to absorb water
WHAT CAUSES EFFLORESCENCE?
Efflorescence occurs when calcium hydroxide (lime) formed in the hydration reaction of portland cement is transported by water to the surface through capillaries in the concrete. There it combines with carbon dioxide from the air to produce insoluble calcium carbonate and water.Apart from presenting a cosmetic outer problem, cleaning and removing the white, powdery scum may cost a lot of time, money and effort.
HOW TO AVOID EFFLORESCENCE?
Understanding how efflorescence works, also provides information on how to prevent it. Cleaning and removing does not cure the problem; it only removes the symptoms. Efflorescence will reappear until precautionary measures affect the conditions under which efflorescence occurs.Adding hydrophobic admixtures during concrete’s mixing process ensures holistic and permanent protection against water penetrating into the concrete structure: Since the reaction takes place inside the matrix of the concrete, the hydrophobic agent becomes an integral part of the concrete mass and thus cannot be removed or worn off later on.
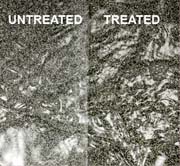
CASE STUDY
Two specimens are tested under conditions necessary for efflorescence. One treated with PROTECT, one non-treated. The specimen treated with PROTECT keeps its appearance whereas the non-treated shows typical white efflorescent salts.


 DEUTSCH
DEUTSCH ENGLISH
ENGLISH NEDERLANDS
NEDERLANDS POLSKI
POLSKI UAE
UAE български
български